

Energy Dispersive X-Ray Spectroscopy (EDS) เป็นการวิเคราะห์องค์ประกอบทางเคมีด้วยสเปก โทรเมตรีรังสีเอกซ์แบบกระจายพลังงานที่ใช้ร่วมกับกล้อง.
- Nomenclature, symbols, units and their usage in spectrochemical analysis-VIII. Nomenclature system for X-ray spectroscopy. Recommendations (1991)Google Scholar
- Jenkins R, Manne R, Robin J, Senemaud C (1991) Part VIII. Nomenclature system for X-ray spectroscopy. Pure Appl Chem 63:735CrossRefGoogle Scholar
- Wollman DA, Irwin KD, Hilton GC, Dulcie LL, Newbury DE, Martinis JM (1997) High-resolution, energy-dispersive microcalorimeter spectrometer for X-ray microanalysis. J Microsc 188:196CrossRefGoogle Scholar
- Wollman DA, Irwin KD, Hilton GC, Dulcie LL, Bergren NF, Newbury DE, Martinis JM (1998) Microcalorimeter EDS with 3 eV energy resolution. In: Proceedings of the 14th international conference on electron microscopy, vol 3, p 573Google Scholar
- Zaluzec NJ (1979) Quantitative X-ray microanalysis. In: Introduction to analytical electron microscopy. Hen JJ, Goldstein JI, Joy DC. (Plenum, New York, p 121)CrossRefGoogle Scholar
- Yang J-M, Shindo D, Takeguchi M, Kawasaki M, Oikawa T (1999) Characterization of microstructure and magnetic domain structure in Sm-Co based permanent magnets by advanced transmission electron microscopy. J Jpn Inst Metals 63:542 (In Japanese)Google Scholar
- Ziebold TO (1967) Precision and sensitivity in microprobe analysis. Anal Chem 39:858CrossRefGoogle Scholar
- Watanabe M, Williams DB (1999) Atomic-level detection by X-ray microanalysis in the analytical electron microscope. Ultramicroscopy 78:89CrossRefGoogle Scholar
- Kawasaki M, Oikawa T, Ibe K, Park K-H, Shiojiri M (1998) EDS elemental mapping of a DRAM with an FE-TEM. J Electron Microsc 47:335CrossRefGoogle Scholar
- Cliff G, Lorimer GW (1975) The quantitative analysis of thin specimens. J Microsc 103:203CrossRefGoogle Scholar
- Schreiber TP, Wims AM (1981) A quantitative Xray microanalysis thin film method using K-, L-, and M-lines. Ultramicroscopy 6:323Google Scholar
- Goldstein JI, Williams DB, Cliff G (1986) Quantitative X-ray analysis. In: Joy DC, Romig AD Jr, Goldstein JI (eds) Principles of analytical electron microscopy. (Plenum, New York, p 155)Google Scholar
- Mott NF, Massey HSW (1949) The theory of atomic collisions, 2nd edn. Oxford University Press, London, p 243zbMATHGoogle Scholar
- Green M, Cosslett VE (1961) The efficiency of production of characteristic X-radiation in thick targets of a pure element. Proc Phys Soc 78:1206ADSCrossRefGoogle Scholar
- Horita Z (1998) Quantitative X-ray microanalysis in analytical electron microscopy. Mater Trans JIM 39:947Google Scholar
- Überall H (1956) High-energy interference effect of bremsstrahlung and pair production in crystals. Phys Rev 103:1055ADSCrossRefGoogle Scholar
- Barbiellini G, Bologna G, Diambrini G, Murtas GP (1962) Experimental evidence for a quasi-monochromatic bremsstrahlung intensity from the Frascati 1-GeV electronsynchrotron. Phys Rev Lett 8:454ADSCrossRefGoogle Scholar
- Spence JCH, Reese G, Yamamoto N, Kurizki G (1983) Coherent bremsstrahlung peaks in X-ray microanalysis spectra. Phil Mag B48:L39CrossRefGoogle Scholar
- Reese GM, Spence JCH, Yamamoto N (1984) Coherent bremsstrahlung from kilovolt electrons in zone axis orientations. Phil Mag A49:697ADSGoogle Scholar
- Spence JCH, Reese G (1986) Pendellösung radiation and coherent bremsstrahlung. Acta Cryst A42: 577Google Scholar
- Satoh T, Otsuki E, Shindo D (1998) Coherent bremsstrahlung in ferrite observed by an analytical transmission electron microscope. J Electron Microsc 47:345CrossRefGoogle Scholar
- Shindo D, Hiraga K, Williams T, Hirabayashi M, Inoue A, Masumoto T (1989) Electron channelling effect in an Al-Fe-Cu quasicrystal. Jpn J Appl Phys 28:L688ADSCrossRefGoogle Scholar
- Cowley JM (1964) The derivation of structural information from absorption effects in X-ray diffraction. Acta Cryst 17:33CrossRefGoogle Scholar
- Batterman BW (1969) Detection of foreign atom sites by their X-ray fluorescence scattering. Phys Rev Lett 22:703ADSCrossRefGoogle Scholar
- Spence JCH, Taftø J (1983) ALCHEMI: a new technique for locating atoms in small crystals. J Microsc 130:147CrossRefGoogle Scholar
- Shindo D, Hirabayashi M, Kawabata T, Kikuchi M (1986) A channelling enhanced microanalysis on niobium atom location in an Al-43 %Ti-2 %Nb intermetallic compound. J Electron Microsc 35:409Google Scholar
- Shindo D, Chiba A, Hiraga K, Hanada S (1991) Electron channelling enhanced microanalysis of intermetallic compounds. In: Izumi O (ed) Proceedings of the International Symposium on Intermetallic Compounds, p 87Google Scholar
- Horita Z, Matsumura S, Baba T (1995) General formulation for ALCHEMI. Ultramicroscopy 58:327CrossRefGoogle Scholar
- Rossouw CJ, Forwood CT, Gibson MA, Miller PR (1996) Statistical ALCHEMI: general formulation and method with application to Ti-Al ternary alloys. Phil Mag A74:57ADSGoogle Scholar
- Shindo D, Kikuchi M, Hirabayashi M, Hanada S, Izumi O (1988) Site determination of Fe, Co and Cr atoms added in Ni3AI by electron channelling enhanced microanalysis. Trans Jpn Inst Metall 29:956Google Scholar
- Chiba A, Shindo D, Hanada S (1991) Site occupation determination of Pd in Ni3Al by ALCHEMI. Acta Metall Mater 39:13CrossRefGoogle Scholar
- Nakata Y, Tadaki T, Shimizu K (1991) Atom location of the third element in Ti-Ni-X shape memory alloys determined by the electron channelling enhanced microanalysis. Mater Trans JIM 32:580Google Scholar
- Spence JCH, Graham RJ, Shindo D (1986) Cold ALCHEMI: impurity atom site location and the temperature dependance of dechannelling. Mater Res Soc Symp Proc 62:153CrossRefGoogle Scholar
- Okaniwa H, Shindo D, Yoshida M, Takasugi T (1999) Determination of site occupancy of additives X (X = V, Mo, W and Ti) in the Nb-Cr-X Laves phase by ALCHEMI. Acta Mater 47:1987CrossRefGoogle Scholar
- Gjønnes J, Høier R (1971) The application of nonsystematic many-beam dynamical effects to structure factor determination. Acta Cryst A27:313Google Scholar
- Matsumura S, Morimura T, Oki K (1991) An analytical electron diffraction technique for the determination of long-range order parameters in multi-component ordered alloys. Mater Trans JIM 32:905Google Scholar
- Bentley J (1986) Axial electron channeling microanalysis of Ll2 ordered alloys. In: Proceedings of the 11th International Congress on Electron Microscopy, Kyoto, vol 1, p 551Google Scholar
- Pennycook SJ (1985) Electron channeling analysis and Z-contrast imaging of dopants in semiconductors. In: Bailey GW (ed) Proceedings of the 43rd annual EMSA meeting. San Francisco Press, San Francisco, p 296Google Scholar
- Pennycook SJ (1988) Delocalization corrections for electron channeling analysis. Ultramicroscopy 26:239CrossRefGoogle Scholar
| Condensed matter experiments |
|---|
| ARPES |
| ACAR |
| Neutron scattering |
| X-ray spectroscopy |
| Quantum oscillations |
| Scanning tunneling microscopy |
X-ray spectroscopy is a general term for several spectroscopic techniques for characterization of materials by using x-ray excitation.[1]
Characteristic X-ray spectroscopy[edit]
When an electron from the inner shell of an atom is excited by the energy of a photon, it moves to a higher energy level. When it returns to the low energy level, the energy which it previously gained by the excitation is emitted as a photon which has a wavelength that is characteristic for the element (there could be several characteristic wavelengths per element). Analysis of the X-ray emission spectrum produces qualitative results about the elemental composition of the specimen. Comparison of the specimen's spectrum with the spectra of samples of known composition produces quantitative results (after some mathematical corrections for absorption, fluorescence and atomic number). Atoms can be excited by a high-energy beam of charged particles such as electrons (in an electron microscope for example), protons (see PIXE) or a beam of X-rays (see X-ray fluorescence, or XRF or also recently in transmission XRT). These methods enable elements from the entire periodic table to be analysed, with the exception of H, He and Li.In electron microscopy an electron beam excites X-rays; there are two main techniques for analysis of spectra of characteristic X-ray radiation: energy-dispersive X-ray spectroscopy (EDS) and wavelength dispersive X-ray spectroscopy (WDS). In X-Ray Transmission (XRT), the equivalent atomic composition (Zeff) is captured based on photoelectric and Compton effects.
Energy-dispersive X-ray spectroscopy[edit]
In an energy-dispersive X-ray spectrometer, a semiconductor detector measures energy of incoming photons. To maintain detector integrity and resolution it should be cooled with liquid nitrogen or by Peltier cooling. EDS is widely employed in electron microscopes (where imaging rather than spectroscopy is a main task) and in cheaper and/or portable XRF units.[citation needed]
Wavelength-dispersive X-ray spectroscopy[edit]
In a wavelength-dispersive X-ray spectrometer, a single crystal diffracts the photons according to Bragg's law, which are then collected by a detector. By moving the diffraction crystal and detector relative to each other, a wide region of the spectrum can be observed. To observe a large spectral range, three of four different single crystals may be needed. In contrast to EDS, WDS is a method of sequential spectrum acquisition. While WDS is slower than EDS and more sensitive to the positioning of the sample in the spectrometer, it has superior spectral resolution and sensitivity. WDS is widely used in microprobes (where X-ray microanalysis is the main task) and in XRF; it is widely used in the field of X-ray diffraction to calculate various data such as interplanar spacing and wavelength of the incident X-ray using Bragg's law.
X-ray emission spectroscopy[edit]
The father-and-son scientific team of William Lawrence Bragg and William Henry Bragg, who were 1915 Nobel Prize Winners, were the original pioneers in developing X-ray emission spectroscopy. Jointly they measured the X-ray wavelengths of many elements to high precision, using high-energy electrons as excitation source. The cathode ray tube or an x-ray tube[2] was the method used to pass electrons through a crystal of numerous elements. They also painstakingly produced numerous diamond-ruled glass diffraction gratings for their spectrometers. The law of diffraction of a crystal is called Bragg's law in their honor.
Intense and wavelength-tunable X-rays are now typically generated with synchrotrons. In a material, the X-rays may suffer an energy loss compared to the incoming beam. This energy loss of the re-emerging beam reflects an internal excitation of the atomic system, an X-ray analogue to the well-known Raman spectroscopy that is widely used in the optical region.
In the X-ray region there is sufficient energy to probe changes in the electronic state (transitions between orbitals; this is in contrast with the optical region, where the energy loss is often due to changes in the state of the rotational or vibrational degrees of freedom). For instance, in the ultra soft X-ray region (below about 1 keV), crystal field excitations give rise to the energy loss.
The photon-in-photon-out process may be thought of as a scattering event. When the x-ray energy corresponds to the binding energy of a core-level electron, this scattering process is resonantly enhanced by many orders of magnitude. This type of X-ray emission spectroscopy is often referred to as resonant inelastic X-ray scattering (RIXS).
Due to the wide separation of orbital energies of the core levels, it is possible to select a certain atom of interest. The small spatial extent of core level orbitals forces the RIXS process to reflect the electronic structure in close vicinity of the chosen atom. Thus, RIXS experiments give valuable information about the local electronic structure of complex systems, and theoretical calculations are relatively simple to perform.
Instrumentation[edit]
There exist several efficient designs for analyzing an X-ray emission spectrum in the ultra soft X-ray region. The figure of merit for such instruments is the spectral throughput, i.e. the product of detected intensity and spectral resolving power. Usually, it is possible to change these parameters within a certain range while keeping their product constant.
Grating spectrometers[edit]
Usually X-ray diffraction in spectrometers is achieved on crystals, but in Grating spectrometers, the X-rays emerging from a sample must pass a source-defining slit, then optical elements (mirrors and/or gratings) disperse them by diffraction according to their wavelength and, finally, a detector is placed at their focal points.
Spherical grating mounts[edit]
Henry Augustus Rowland (1848–1901) devised an instrument that allowed the use of a single optical element that combines diffraction and focusing: a spherical grating. Reflectivity of X-rays is low, regardless of the used material and therefore, grazing incidence upon the grating is necessary. X-ray beams impinging on a smooth surface at a few degrees glancing angle of incidence undergo external total reflection which is taken advantage of to enhance the instrumental efficiency substantially.
Denoted by R the radius of a spherical grating. Imagine a circle with half the radius R tangent to the center of the grating surface. This small circle is called the Rowland circle. If the entrance slit is anywhere on this circle, then a beam passing the slit and striking the grating will be split into a specularly reflected beam, and beams of all diffraction orders, that come into focus at certain points on the same circle.
Plane grating mounts[edit]
Similar to optical spectrometers, a plane grating spectrometer first needs optics that turns the divergent rays emitted by the x-ray source into a parallel beam. This may be achieved by using a parabolic mirror. The parallel rays emerging from this mirror strike a plane grating (with constant groove distance) at the same angle and are diffracted according to their wavelength. A second parabolic mirror then collects the diffracted rays at a certain angle and creates an image on a detector. A spectrum within a certain wavelength range can be recorded simultaneously by using a two-dimensional position-sensitive detector such as a microchannel photomultiplier plate or an X-ray sensitive CCD chip (film plates are also possible to use).
Interferometers[edit]
Instead of using the concept of multiple beam interference that gratings produce, the two rays may simply interfere. By recording the intensity of two such co-linearly at some fixed point and changing their relative phase one obtains an intensity spectrum as a function of path length difference. One can show that this is equivalent to a Fourier transformed spectrum as a function of frequency. The highest recordable frequency of such a spectrum is dependent on the minimum step size chosen in the scan and the frequency resolution (i.e. how well a certain wave can be defined in terms of its frequency) depends on the maximum path length difference achieved. The latter feature allows a much more compact design for achieving high resolution than for a grating spectrometer because x-ray wavelengths are small compared to attainable path length differences.
Early history of X-ray spectroscopy in the U.S.[edit]
Philips Gloeilampen Fabrieken, headquartered in Eindhoven in the Netherlands, got its start as a manufacturer of light bulbs, but quickly evolved until it is now one of the leading manufacturers of electrical apparatus, electronics, and related products including X-ray equipment. It also has had one of the world's largest R&D labs. In 1940, the Netherlands was overrun by Hitler’s Germany. The company was able to transfer a substantial sum of money to a company that it set up as an R&D laboratory in an estate in Irvington on the Hudson in NY. As an extension to their work on light bulbs, the Dutch company had developed a line of X-ray tubes for medical applications that were powered by transformers. These X-ray tubes could also be used in scientific X-ray instrumentations, but there was very little commercial demand for the latter. As a result, management decided to try to develop this market and they set up development groups in their research labs in both Holland and the U.S.
They hired Dr. Ira Duffendack, a professor at University of Michigan and a world expert on infrared research to head the lab and to hire a staff. In 1951 he hired Dr. David Miller as Assistant Director of Research. Dr. Miller had done research on X-ray instrumentation at Washington University in St. Louis. Dr. Duffendack also hired Dr. Bill Parish, a well known researcher in X-ray diffraction, to head up the section of the lab on X-ray instrumental development. X-ray diffraction units were widely used in academic research departments to do crystal analysis. An essential component of a diffraction unit was a very accurate angle measuring device known as a goniometer. Such units were not commercially available, so each investigator had do try to make their own. Dr Parrish decided this would be a good device to use to generate an instrumental market, so his group designed and learned how to manufacture a goniometer. This market developed quickly and, with the readily available tubes and power supplies, a complete diffraction unit was made available and was successfully marketed.
The U.S. management did not want the laboratory to be converted to a manufacturing unit so it decided to set up a commercial unit to further develop the X-ray instrumentation market. In 1953 Norelco Electronics was established in Mount Vernon, NY, dedicated to the sale and support of X-ray instrumentation. It included a sales staff, a manufacturing group, an engineering department and an applications lab. Dr. Miller was transferred from the lab to head up the engineering department. The sales staff sponsored three schools a year, one in Mount Vernon, one in Denver, and one in San Francisco. The week-long school curricula reviewed the basics of X-ray instrumentation and the specific application of Norelco products. The faculty were members of the engineering department and academic consultants. The schools were well attended by academic and industrial R&D scientists. The engineering department was also a new product development group. It added an X-ray spectrograph to the product line very quickly and contributed other related products for the next 8 years.
The applications lab was an essential sales tool. When the spectrograph was introduced as a quick and accurate analytical chemistry device, it met with widespread skepticism. All research facilities had a chemistry department and analytical analysis was done by “wet chemistry” methods. The idea of doing this analysis by physics instrumentation was considered suspect. To overcome this bias, the salesman would ask a prospective customer for a task the customer was doing by “wet methods”. The task would be given to the applications lab and they would demonstrate how accurately and quickly it could be done using the X-ray units. This proved to be a very strong sales tool, particularly when the results were published in the Norelco Reporter, a technical journal issued monthly by the company with wide distribution to commercial and academic institutions.
An X-ray spectrograph consists of a high voltage power supply (50 kV or 100 kV), a broad band X-ray tube, usually with a tungsten anode and a beryllium window, a specimen holder, an analyzing crystal, a goniometer, and an X-ray detector device. These are arranged as shown in Fig. 1.
Fig. 1
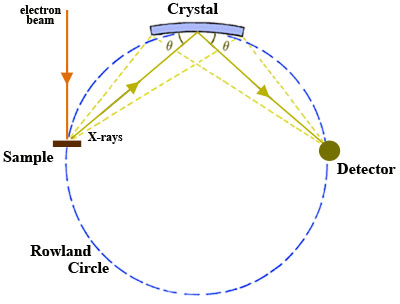
The continuous X-spectrum emitted from the tube irradiates the specimen and excites the characteristic spectral X-ray lines in the specimen. Each of the 92 elements emits a characteristic spectrum. Unlike the optical spectrum, the X-ray spectrum is quite simple. The strongest line, usually the Kalpha line, but sometimes the Lalpha line, suffices to identify the element. The existence of a particular line betrays the existence of an element, and the intensity is proportional to the amount of the particular element in the specimen. The characteristic lines are reflected from a crystal, the analyzer, under an angle that is given by the Bragg condition. The crystal samples all the diffraction angles theta by rotation, while the detector rotates over the corresponding angle 2-theta. With a sensitive detector, the X-ray photons are counted individually. By stepping the detectors along the angle, and leaving it in position for a known time, the number of counts at each angular position gives the line intensity. These counts may be plotted on a curve by an appropriate display unit. The characteristic X-rays come out at specific angles, and since the angular position for every X-ray spectral line is known and recorded, it is easy to find the sample's composition.
A chart for a scan of a Molybdenum specimen is shown in Fig. 2. The tall peak on the left side is the characteristic alpha line at a two theta of 12 degrees. Second and third order lines also appear.
Fig. 2
Aja kona 3 driver mac. AJA is dedicated to ensuring your success with our products. Technical Support is available free of charge and our team will work with you to help answer questions or resolve any issues. KONA MAC System Configuration KONA for Mac Supported System Configurations. The AJA engineering, QA and technical support teams have determined the optimum slot placement for the AJA KONA cards in the Apple MacPro. In order to assure proper functionality, the card should be seated in the slot specified by AJA. KONA 3 Support. Please note, the last supported macOS is Yosemite. The KONA 3 will not work on any OS past Yosemite. The last supported Windows OS is Windows 7.
Since the alpha line is often the only line of interest in many industrial applications, the final device in the Norelco X- ray spectrographic instrument line was the Autrometer. This device could be programmed to automatically read at any desired two theta angle for any desired time interval.
Soon after the Autrometer was introduced, Philips decided to stop marketing X-ray instruments developed in both the U.S. and Europe and settled on offering only the Eindhoven line of instruments.
In 1961, during the development of the Autrometer, Norelco was given a sub-contract from the Jet Propulsion Lab. The Lab was working on the instrument package for the Surveyor spaceship. The composition of the moon’s surface was of major interest and the use of an X-ray detection instrument was viewed as a possible solution. Working with a power limit of 30 watts was very challenging, but a device was delivered but it wasn’t used. Later NASA developments did lead to an X-ray spectrographic unit that did make the desired moon soil analysis.
The Norelco efforts faded but the use of X-ray spectroscopy in units known as XRF instruments continued to grow. With a boost from NASA, units were finally reduced to handheld size and are seeing widespread use. Units are available from Bruker, Thermo Scientific, Elvatech Ltd. and SPECTRA.
Other types of X-ray spectroscopy[edit]
See also[edit]
References[edit]
- ^'x ray spectroscopy'(PDF).
- ^Fonda, Gorton R.; Collins, George B. (1931-01-01). 'THE CATHODE RAY TUBE IN X-RAY SPECTROSCOPY AND QUANTITATIVE ANALYSIS'. Journal of the American Chemical Society. 53 (1): 113–125. doi:10.1021/ja01352a017. ISSN0002-7863.
- Author: admin
- Category: Category

Energy Dispersive X-Ray Spectroscopy (EDS) เป็นการวิเคราะห์องค์ประกอบทางเคมีด้วยสเปก โทรเมตรีรังสีเอกซ์แบบกระจายพลังงานที่ใช้ร่วมกับกล้อง.
- Nomenclature, symbols, units and their usage in spectrochemical analysis-VIII. Nomenclature system for X-ray spectroscopy. Recommendations (1991)Google Scholar
- Jenkins R, Manne R, Robin J, Senemaud C (1991) Part VIII. Nomenclature system for X-ray spectroscopy. Pure Appl Chem 63:735CrossRefGoogle Scholar
- Wollman DA, Irwin KD, Hilton GC, Dulcie LL, Newbury DE, Martinis JM (1997) High-resolution, energy-dispersive microcalorimeter spectrometer for X-ray microanalysis. J Microsc 188:196CrossRefGoogle Scholar
- Wollman DA, Irwin KD, Hilton GC, Dulcie LL, Bergren NF, Newbury DE, Martinis JM (1998) Microcalorimeter EDS with 3 eV energy resolution. In: Proceedings of the 14th international conference on electron microscopy, vol 3, p 573Google Scholar
- Zaluzec NJ (1979) Quantitative X-ray microanalysis. In: Introduction to analytical electron microscopy. Hen JJ, Goldstein JI, Joy DC. (Plenum, New York, p 121)CrossRefGoogle Scholar
- Yang J-M, Shindo D, Takeguchi M, Kawasaki M, Oikawa T (1999) Characterization of microstructure and magnetic domain structure in Sm-Co based permanent magnets by advanced transmission electron microscopy. J Jpn Inst Metals 63:542 (In Japanese)Google Scholar
- Ziebold TO (1967) Precision and sensitivity in microprobe analysis. Anal Chem 39:858CrossRefGoogle Scholar
- Watanabe M, Williams DB (1999) Atomic-level detection by X-ray microanalysis in the analytical electron microscope. Ultramicroscopy 78:89CrossRefGoogle Scholar
- Kawasaki M, Oikawa T, Ibe K, Park K-H, Shiojiri M (1998) EDS elemental mapping of a DRAM with an FE-TEM. J Electron Microsc 47:335CrossRefGoogle Scholar
- Cliff G, Lorimer GW (1975) The quantitative analysis of thin specimens. J Microsc 103:203CrossRefGoogle Scholar
- Schreiber TP, Wims AM (1981) A quantitative Xray microanalysis thin film method using K-, L-, and M-lines. Ultramicroscopy 6:323Google Scholar
- Goldstein JI, Williams DB, Cliff G (1986) Quantitative X-ray analysis. In: Joy DC, Romig AD Jr, Goldstein JI (eds) Principles of analytical electron microscopy. (Plenum, New York, p 155)Google Scholar
- Mott NF, Massey HSW (1949) The theory of atomic collisions, 2nd edn. Oxford University Press, London, p 243zbMATHGoogle Scholar
- Green M, Cosslett VE (1961) The efficiency of production of characteristic X-radiation in thick targets of a pure element. Proc Phys Soc 78:1206ADSCrossRefGoogle Scholar
- Horita Z (1998) Quantitative X-ray microanalysis in analytical electron microscopy. Mater Trans JIM 39:947Google Scholar
- Überall H (1956) High-energy interference effect of bremsstrahlung and pair production in crystals. Phys Rev 103:1055ADSCrossRefGoogle Scholar
- Barbiellini G, Bologna G, Diambrini G, Murtas GP (1962) Experimental evidence for a quasi-monochromatic bremsstrahlung intensity from the Frascati 1-GeV electronsynchrotron. Phys Rev Lett 8:454ADSCrossRefGoogle Scholar
- Spence JCH, Reese G, Yamamoto N, Kurizki G (1983) Coherent bremsstrahlung peaks in X-ray microanalysis spectra. Phil Mag B48:L39CrossRefGoogle Scholar
- Reese GM, Spence JCH, Yamamoto N (1984) Coherent bremsstrahlung from kilovolt electrons in zone axis orientations. Phil Mag A49:697ADSGoogle Scholar
- Spence JCH, Reese G (1986) Pendellösung radiation and coherent bremsstrahlung. Acta Cryst A42: 577Google Scholar
- Satoh T, Otsuki E, Shindo D (1998) Coherent bremsstrahlung in ferrite observed by an analytical transmission electron microscope. J Electron Microsc 47:345CrossRefGoogle Scholar
- Shindo D, Hiraga K, Williams T, Hirabayashi M, Inoue A, Masumoto T (1989) Electron channelling effect in an Al-Fe-Cu quasicrystal. Jpn J Appl Phys 28:L688ADSCrossRefGoogle Scholar
- Cowley JM (1964) The derivation of structural information from absorption effects in X-ray diffraction. Acta Cryst 17:33CrossRefGoogle Scholar
- Batterman BW (1969) Detection of foreign atom sites by their X-ray fluorescence scattering. Phys Rev Lett 22:703ADSCrossRefGoogle Scholar
- Spence JCH, Taftø J (1983) ALCHEMI: a new technique for locating atoms in small crystals. J Microsc 130:147CrossRefGoogle Scholar
- Shindo D, Hirabayashi M, Kawabata T, Kikuchi M (1986) A channelling enhanced microanalysis on niobium atom location in an Al-43 %Ti-2 %Nb intermetallic compound. J Electron Microsc 35:409Google Scholar
- Shindo D, Chiba A, Hiraga K, Hanada S (1991) Electron channelling enhanced microanalysis of intermetallic compounds. In: Izumi O (ed) Proceedings of the International Symposium on Intermetallic Compounds, p 87Google Scholar
- Horita Z, Matsumura S, Baba T (1995) General formulation for ALCHEMI. Ultramicroscopy 58:327CrossRefGoogle Scholar
- Rossouw CJ, Forwood CT, Gibson MA, Miller PR (1996) Statistical ALCHEMI: general formulation and method with application to Ti-Al ternary alloys. Phil Mag A74:57ADSGoogle Scholar
- Shindo D, Kikuchi M, Hirabayashi M, Hanada S, Izumi O (1988) Site determination of Fe, Co and Cr atoms added in Ni3AI by electron channelling enhanced microanalysis. Trans Jpn Inst Metall 29:956Google Scholar
- Chiba A, Shindo D, Hanada S (1991) Site occupation determination of Pd in Ni3Al by ALCHEMI. Acta Metall Mater 39:13CrossRefGoogle Scholar
- Nakata Y, Tadaki T, Shimizu K (1991) Atom location of the third element in Ti-Ni-X shape memory alloys determined by the electron channelling enhanced microanalysis. Mater Trans JIM 32:580Google Scholar
- Spence JCH, Graham RJ, Shindo D (1986) Cold ALCHEMI: impurity atom site location and the temperature dependance of dechannelling. Mater Res Soc Symp Proc 62:153CrossRefGoogle Scholar
- Okaniwa H, Shindo D, Yoshida M, Takasugi T (1999) Determination of site occupancy of additives X (X = V, Mo, W and Ti) in the Nb-Cr-X Laves phase by ALCHEMI. Acta Mater 47:1987CrossRefGoogle Scholar
- Gjønnes J, Høier R (1971) The application of nonsystematic many-beam dynamical effects to structure factor determination. Acta Cryst A27:313Google Scholar
- Matsumura S, Morimura T, Oki K (1991) An analytical electron diffraction technique for the determination of long-range order parameters in multi-component ordered alloys. Mater Trans JIM 32:905Google Scholar
- Bentley J (1986) Axial electron channeling microanalysis of Ll2 ordered alloys. In: Proceedings of the 11th International Congress on Electron Microscopy, Kyoto, vol 1, p 551Google Scholar
- Pennycook SJ (1985) Electron channeling analysis and Z-contrast imaging of dopants in semiconductors. In: Bailey GW (ed) Proceedings of the 43rd annual EMSA meeting. San Francisco Press, San Francisco, p 296Google Scholar
- Pennycook SJ (1988) Delocalization corrections for electron channeling analysis. Ultramicroscopy 26:239CrossRefGoogle Scholar
| Condensed matter experiments |
|---|
| ARPES |
| ACAR |
| Neutron scattering |
| X-ray spectroscopy |
| Quantum oscillations |
| Scanning tunneling microscopy |
X-ray spectroscopy is a general term for several spectroscopic techniques for characterization of materials by using x-ray excitation.[1]
Characteristic X-ray spectroscopy[edit]
When an electron from the inner shell of an atom is excited by the energy of a photon, it moves to a higher energy level. When it returns to the low energy level, the energy which it previously gained by the excitation is emitted as a photon which has a wavelength that is characteristic for the element (there could be several characteristic wavelengths per element). Analysis of the X-ray emission spectrum produces qualitative results about the elemental composition of the specimen. Comparison of the specimen's spectrum with the spectra of samples of known composition produces quantitative results (after some mathematical corrections for absorption, fluorescence and atomic number). Atoms can be excited by a high-energy beam of charged particles such as electrons (in an electron microscope for example), protons (see PIXE) or a beam of X-rays (see X-ray fluorescence, or XRF or also recently in transmission XRT). These methods enable elements from the entire periodic table to be analysed, with the exception of H, He and Li.In electron microscopy an electron beam excites X-rays; there are two main techniques for analysis of spectra of characteristic X-ray radiation: energy-dispersive X-ray spectroscopy (EDS) and wavelength dispersive X-ray spectroscopy (WDS). In X-Ray Transmission (XRT), the equivalent atomic composition (Zeff) is captured based on photoelectric and Compton effects.
Energy-dispersive X-ray spectroscopy[edit]
In an energy-dispersive X-ray spectrometer, a semiconductor detector measures energy of incoming photons. To maintain detector integrity and resolution it should be cooled with liquid nitrogen or by Peltier cooling. EDS is widely employed in electron microscopes (where imaging rather than spectroscopy is a main task) and in cheaper and/or portable XRF units.[citation needed]
Wavelength-dispersive X-ray spectroscopy[edit]
In a wavelength-dispersive X-ray spectrometer, a single crystal diffracts the photons according to Bragg's law, which are then collected by a detector. By moving the diffraction crystal and detector relative to each other, a wide region of the spectrum can be observed. To observe a large spectral range, three of four different single crystals may be needed. In contrast to EDS, WDS is a method of sequential spectrum acquisition. While WDS is slower than EDS and more sensitive to the positioning of the sample in the spectrometer, it has superior spectral resolution and sensitivity. WDS is widely used in microprobes (where X-ray microanalysis is the main task) and in XRF; it is widely used in the field of X-ray diffraction to calculate various data such as interplanar spacing and wavelength of the incident X-ray using Bragg's law.
X-ray emission spectroscopy[edit]
The father-and-son scientific team of William Lawrence Bragg and William Henry Bragg, who were 1915 Nobel Prize Winners, were the original pioneers in developing X-ray emission spectroscopy. Jointly they measured the X-ray wavelengths of many elements to high precision, using high-energy electrons as excitation source. The cathode ray tube or an x-ray tube[2] was the method used to pass electrons through a crystal of numerous elements. They also painstakingly produced numerous diamond-ruled glass diffraction gratings for their spectrometers. The law of diffraction of a crystal is called Bragg's law in their honor.
Intense and wavelength-tunable X-rays are now typically generated with synchrotrons. In a material, the X-rays may suffer an energy loss compared to the incoming beam. This energy loss of the re-emerging beam reflects an internal excitation of the atomic system, an X-ray analogue to the well-known Raman spectroscopy that is widely used in the optical region.
In the X-ray region there is sufficient energy to probe changes in the electronic state (transitions between orbitals; this is in contrast with the optical region, where the energy loss is often due to changes in the state of the rotational or vibrational degrees of freedom). For instance, in the ultra soft X-ray region (below about 1 keV), crystal field excitations give rise to the energy loss.
The photon-in-photon-out process may be thought of as a scattering event. When the x-ray energy corresponds to the binding energy of a core-level electron, this scattering process is resonantly enhanced by many orders of magnitude. This type of X-ray emission spectroscopy is often referred to as resonant inelastic X-ray scattering (RIXS).
Due to the wide separation of orbital energies of the core levels, it is possible to select a certain atom of interest. The small spatial extent of core level orbitals forces the RIXS process to reflect the electronic structure in close vicinity of the chosen atom. Thus, RIXS experiments give valuable information about the local electronic structure of complex systems, and theoretical calculations are relatively simple to perform.
Instrumentation[edit]
There exist several efficient designs for analyzing an X-ray emission spectrum in the ultra soft X-ray region. The figure of merit for such instruments is the spectral throughput, i.e. the product of detected intensity and spectral resolving power. Usually, it is possible to change these parameters within a certain range while keeping their product constant.
Grating spectrometers[edit]
Usually X-ray diffraction in spectrometers is achieved on crystals, but in Grating spectrometers, the X-rays emerging from a sample must pass a source-defining slit, then optical elements (mirrors and/or gratings) disperse them by diffraction according to their wavelength and, finally, a detector is placed at their focal points.
Spherical grating mounts[edit]
Henry Augustus Rowland (1848–1901) devised an instrument that allowed the use of a single optical element that combines diffraction and focusing: a spherical grating. Reflectivity of X-rays is low, regardless of the used material and therefore, grazing incidence upon the grating is necessary. X-ray beams impinging on a smooth surface at a few degrees glancing angle of incidence undergo external total reflection which is taken advantage of to enhance the instrumental efficiency substantially.
Denoted by R the radius of a spherical grating. Imagine a circle with half the radius R tangent to the center of the grating surface. This small circle is called the Rowland circle. If the entrance slit is anywhere on this circle, then a beam passing the slit and striking the grating will be split into a specularly reflected beam, and beams of all diffraction orders, that come into focus at certain points on the same circle.
Plane grating mounts[edit]
Similar to optical spectrometers, a plane grating spectrometer first needs optics that turns the divergent rays emitted by the x-ray source into a parallel beam. This may be achieved by using a parabolic mirror. The parallel rays emerging from this mirror strike a plane grating (with constant groove distance) at the same angle and are diffracted according to their wavelength. A second parabolic mirror then collects the diffracted rays at a certain angle and creates an image on a detector. A spectrum within a certain wavelength range can be recorded simultaneously by using a two-dimensional position-sensitive detector such as a microchannel photomultiplier plate or an X-ray sensitive CCD chip (film plates are also possible to use).
Interferometers[edit]
Instead of using the concept of multiple beam interference that gratings produce, the two rays may simply interfere. By recording the intensity of two such co-linearly at some fixed point and changing their relative phase one obtains an intensity spectrum as a function of path length difference. One can show that this is equivalent to a Fourier transformed spectrum as a function of frequency. The highest recordable frequency of such a spectrum is dependent on the minimum step size chosen in the scan and the frequency resolution (i.e. how well a certain wave can be defined in terms of its frequency) depends on the maximum path length difference achieved. The latter feature allows a much more compact design for achieving high resolution than for a grating spectrometer because x-ray wavelengths are small compared to attainable path length differences.
Early history of X-ray spectroscopy in the U.S.[edit]
Philips Gloeilampen Fabrieken, headquartered in Eindhoven in the Netherlands, got its start as a manufacturer of light bulbs, but quickly evolved until it is now one of the leading manufacturers of electrical apparatus, electronics, and related products including X-ray equipment. It also has had one of the world's largest R&D labs. In 1940, the Netherlands was overrun by Hitler’s Germany. The company was able to transfer a substantial sum of money to a company that it set up as an R&D laboratory in an estate in Irvington on the Hudson in NY. As an extension to their work on light bulbs, the Dutch company had developed a line of X-ray tubes for medical applications that were powered by transformers. These X-ray tubes could also be used in scientific X-ray instrumentations, but there was very little commercial demand for the latter. As a result, management decided to try to develop this market and they set up development groups in their research labs in both Holland and the U.S.
They hired Dr. Ira Duffendack, a professor at University of Michigan and a world expert on infrared research to head the lab and to hire a staff. In 1951 he hired Dr. David Miller as Assistant Director of Research. Dr. Miller had done research on X-ray instrumentation at Washington University in St. Louis. Dr. Duffendack also hired Dr. Bill Parish, a well known researcher in X-ray diffraction, to head up the section of the lab on X-ray instrumental development. X-ray diffraction units were widely used in academic research departments to do crystal analysis. An essential component of a diffraction unit was a very accurate angle measuring device known as a goniometer. Such units were not commercially available, so each investigator had do try to make their own. Dr Parrish decided this would be a good device to use to generate an instrumental market, so his group designed and learned how to manufacture a goniometer. This market developed quickly and, with the readily available tubes and power supplies, a complete diffraction unit was made available and was successfully marketed.
The U.S. management did not want the laboratory to be converted to a manufacturing unit so it decided to set up a commercial unit to further develop the X-ray instrumentation market. In 1953 Norelco Electronics was established in Mount Vernon, NY, dedicated to the sale and support of X-ray instrumentation. It included a sales staff, a manufacturing group, an engineering department and an applications lab. Dr. Miller was transferred from the lab to head up the engineering department. The sales staff sponsored three schools a year, one in Mount Vernon, one in Denver, and one in San Francisco. The week-long school curricula reviewed the basics of X-ray instrumentation and the specific application of Norelco products. The faculty were members of the engineering department and academic consultants. The schools were well attended by academic and industrial R&D scientists. The engineering department was also a new product development group. It added an X-ray spectrograph to the product line very quickly and contributed other related products for the next 8 years.
The applications lab was an essential sales tool. When the spectrograph was introduced as a quick and accurate analytical chemistry device, it met with widespread skepticism. All research facilities had a chemistry department and analytical analysis was done by “wet chemistry” methods. The idea of doing this analysis by physics instrumentation was considered suspect. To overcome this bias, the salesman would ask a prospective customer for a task the customer was doing by “wet methods”. The task would be given to the applications lab and they would demonstrate how accurately and quickly it could be done using the X-ray units. This proved to be a very strong sales tool, particularly when the results were published in the Norelco Reporter, a technical journal issued monthly by the company with wide distribution to commercial and academic institutions.
An X-ray spectrograph consists of a high voltage power supply (50 kV or 100 kV), a broad band X-ray tube, usually with a tungsten anode and a beryllium window, a specimen holder, an analyzing crystal, a goniometer, and an X-ray detector device. These are arranged as shown in Fig. 1.
Fig. 1

The continuous X-spectrum emitted from the tube irradiates the specimen and excites the characteristic spectral X-ray lines in the specimen. Each of the 92 elements emits a characteristic spectrum. Unlike the optical spectrum, the X-ray spectrum is quite simple. The strongest line, usually the Kalpha line, but sometimes the Lalpha line, suffices to identify the element. The existence of a particular line betrays the existence of an element, and the intensity is proportional to the amount of the particular element in the specimen. The characteristic lines are reflected from a crystal, the analyzer, under an angle that is given by the Bragg condition. The crystal samples all the diffraction angles theta by rotation, while the detector rotates over the corresponding angle 2-theta. With a sensitive detector, the X-ray photons are counted individually. By stepping the detectors along the angle, and leaving it in position for a known time, the number of counts at each angular position gives the line intensity. These counts may be plotted on a curve by an appropriate display unit. The characteristic X-rays come out at specific angles, and since the angular position for every X-ray spectral line is known and recorded, it is easy to find the sample's composition.
A chart for a scan of a Molybdenum specimen is shown in Fig. 2. The tall peak on the left side is the characteristic alpha line at a two theta of 12 degrees. Second and third order lines also appear.
Fig. 2
Aja kona 3 driver mac. AJA is dedicated to ensuring your success with our products. Technical Support is available free of charge and our team will work with you to help answer questions or resolve any issues. KONA MAC System Configuration KONA for Mac Supported System Configurations. The AJA engineering, QA and technical support teams have determined the optimum slot placement for the AJA KONA cards in the Apple MacPro. In order to assure proper functionality, the card should be seated in the slot specified by AJA. KONA 3 Support. Please note, the last supported macOS is Yosemite. The KONA 3 will not work on any OS past Yosemite. The last supported Windows OS is Windows 7.
Since the alpha line is often the only line of interest in many industrial applications, the final device in the Norelco X- ray spectrographic instrument line was the Autrometer. This device could be programmed to automatically read at any desired two theta angle for any desired time interval.
Soon after the Autrometer was introduced, Philips decided to stop marketing X-ray instruments developed in both the U.S. and Europe and settled on offering only the Eindhoven line of instruments.
In 1961, during the development of the Autrometer, Norelco was given a sub-contract from the Jet Propulsion Lab. The Lab was working on the instrument package for the Surveyor spaceship. The composition of the moon’s surface was of major interest and the use of an X-ray detection instrument was viewed as a possible solution. Working with a power limit of 30 watts was very challenging, but a device was delivered but it wasn’t used. Later NASA developments did lead to an X-ray spectrographic unit that did make the desired moon soil analysis.
The Norelco efforts faded but the use of X-ray spectroscopy in units known as XRF instruments continued to grow. With a boost from NASA, units were finally reduced to handheld size and are seeing widespread use. Units are available from Bruker, Thermo Scientific, Elvatech Ltd. and SPECTRA.
Other types of X-ray spectroscopy[edit]
See also[edit]
References[edit]
- ^'x ray spectroscopy'(PDF).
- ^Fonda, Gorton R.; Collins, George B. (1931-01-01). 'THE CATHODE RAY TUBE IN X-RAY SPECTROSCOPY AND QUANTITATIVE ANALYSIS'. Journal of the American Chemical Society. 53 (1): 113–125. doi:10.1021/ja01352a017. ISSN0002-7863.
